Data availability statement
Developing new Lithopone formulations, one that enhances the properties of the existing Lithopone is anticipated to boost the demand for Lithopone white pigment during the forecast period. Reinforced Lithopone is one such development, wherein a copolymer is added to the polymerization reaction to yield Lithopone with an increased weather resistance and an anti-ultraviolet property. Moreover, development of nano-scale Lithopone is also anticipated to attract market interest during the forecast period.
Another critical aspect is logistics; given the vast geographical expanse of China, suppliers with efficient distribution networks can ensure timely delivery, reducing potential delays in construction schedules tio2 is a suppliers. They are responsible for maintaining a steady supply chain, managing inventory, and ensuring timely deliveries to meet customer needs. They also play a significant part in research and development, collaborating with manufacturers to innovate new grades and forms of TiO2 that can enhance product performance.
tio2 is a suppliers. They are responsible for maintaining a steady supply chain, managing inventory, and ensuring timely deliveries to meet customer needs. They also play a significant part in research and development, collaborating with manufacturers to innovate new grades and forms of TiO2 that can enhance product performance. Pure titanium dioxide is a fine, white powder that provides a bright, white pigment. Titanium dioxide has been used for a century in a range of industrial and consumer products, including paints, coatings, adhesives, paper, plastics and rubber, printing inks, coated fabrics and textiles, as well as ceramics, floor coverings, roofing materials, cosmetics, toothpaste, soap, water treatment agents, pharmaceuticals, food colorants, automotive products, sunscreen and catalysts.
FAQ – EFSA 2021 safety assessment of titanium dioxide (E171)
Suppliers of titanium dioxide for coatings provide manufacturers with the raw material needed to produce high-quality coatings. These suppliers offer different grades and forms of titanium dioxide to meet the specific requirements of various coatings applications. Whether it is for architectural coatings, automotive coatings, or industrial coatings, suppliers of titanium dioxide play a critical role in ensuring that manufacturers have access to the right materials to produce coatings that meet their performance and aesthetic goals. Titanium dioxide (TiO2) is a versatile and widely used compound with a myriad of applications across diverse industries. Its importance is underpinned by the role it plays in sectors ranging from cosmetics to paints, food, and even solar panels. This has led to a thriving market for titanium dioxide suppliers who cater to these demands, ensuring a consistent and high-quality supply of this crucial material. However, China's Tio2 pigment industry is not without challengesIt doesn’t take much to imagine what they must be doing to our poor skin each day as we layer on our sunscreen, foundation, concealers, eyeshadows & lip sticks which all contain large doses of titanium dioxide.
Overall, titanium dioxide plays a critical role in the paper industry by improving the quality, performance, and appearance of a wide range of paper products. Its unique optical properties make it a valuable additive for enhancing the whiteness, brightness, and opacity of paper, while also providing important functional benefits such as print quality, show-through prevention, and light stability. In addition to its mechanical benefits, titanium dioxide also exhibits photocatalytic propertiesThis article discusses the discovery of phosphorescent lithopone on watercolor drawings by American artist John La Farge dated between 1890 and 1905 and the history of lithopone in the pigment industry in the late 19th and early 20th centuries. Despite having many desirable qualities for use in white watercolor or oil paints, the development of lithopone as an artists’ pigment was hampered by its tendency to darken in sunlight. Its availability to, and adoption by, artists remain unclear, as colormen's trade catalogs were generally not explicit in describing white pigments as containing lithopone. Further, lithopone may be mistaken for lead white during visual examination and its short-lived phosphorescence can be easily missed by the uninformed observer. Phosphorescent lithopone has been documented on only one other work-to-date: a watercolor by Van Gogh. In addition to the history of lithopone's manufacture, the article details the mechanism for its phosphorescence and its identification aided by Raman spectroscopy and spectrofluorimetry.
Both rutile and anatase titanium dioxide factories require strict quality control measures to ensure the purity and consistency of the final product. The production processes involve several stages, including raw material preparation, chemical reactions, particle formation, and finishing. Each stage must be carefully monitored and controlled to ensure the desired properties of the final product.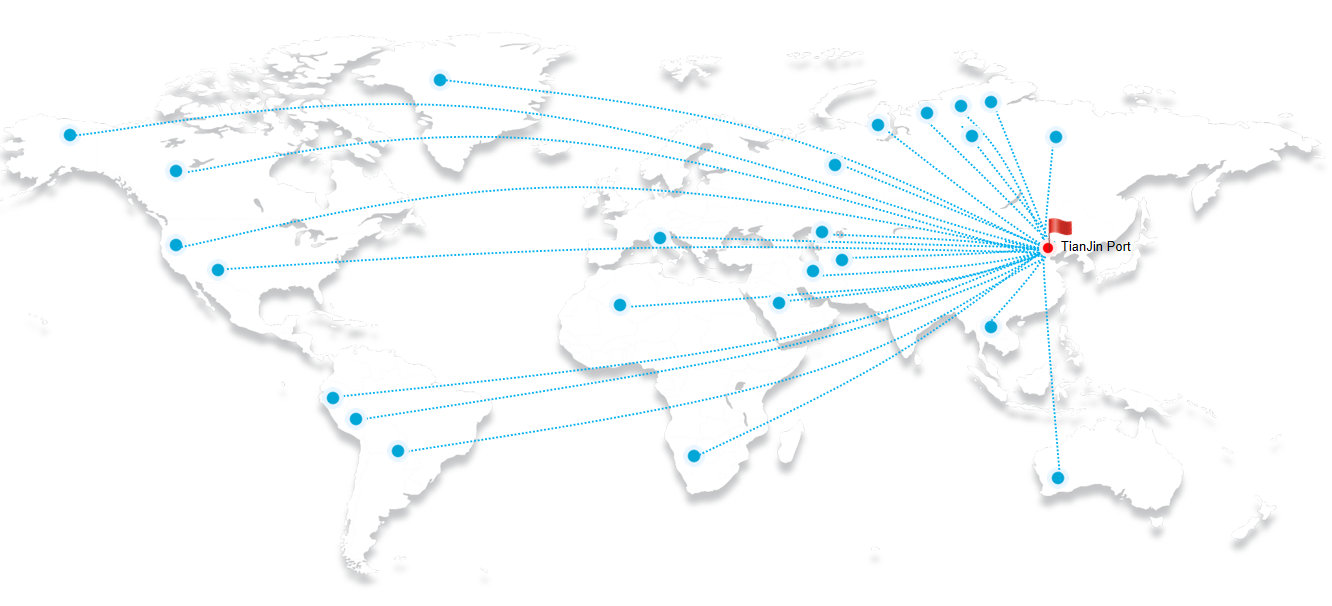 ntr 606 titanium dioxide suppliers. These newcomers are leveraging technological advancements to improve production processes, reduce costs, and enhance product quality. As a result, they are gaining momentum and posing a challenge to the dominant players in the market.
ntr 606 titanium dioxide suppliers. These newcomers are leveraging technological advancements to improve production processes, reduce costs, and enhance product quality. As a result, they are gaining momentum and posing a challenge to the dominant players in the market. 

